ABC in APC
ABC in APC
Prof. Dr. Alaa Nouh M.D.
Prof. of Trop. Med. Menofeya University
Elected International Member of AGA
INTRODUCTION
Argon plasma coagulation (APC) is a non-contact thermal method of hemostasis that has generated much attention and excitement in recent years. It was introduced as an alternative to contact thermal coagulation (heater probe and bipolar cautery) and to existing non-contact technologies (primarily laser). The theoretical advantages of APC include its ease of application, speedy treatment of multiple lesions in the case of angiodysplasias or wide areas (the base of resected polyps or tumor bleeding), safety due to reduced depth of penetration, and lower cost compared to laser.
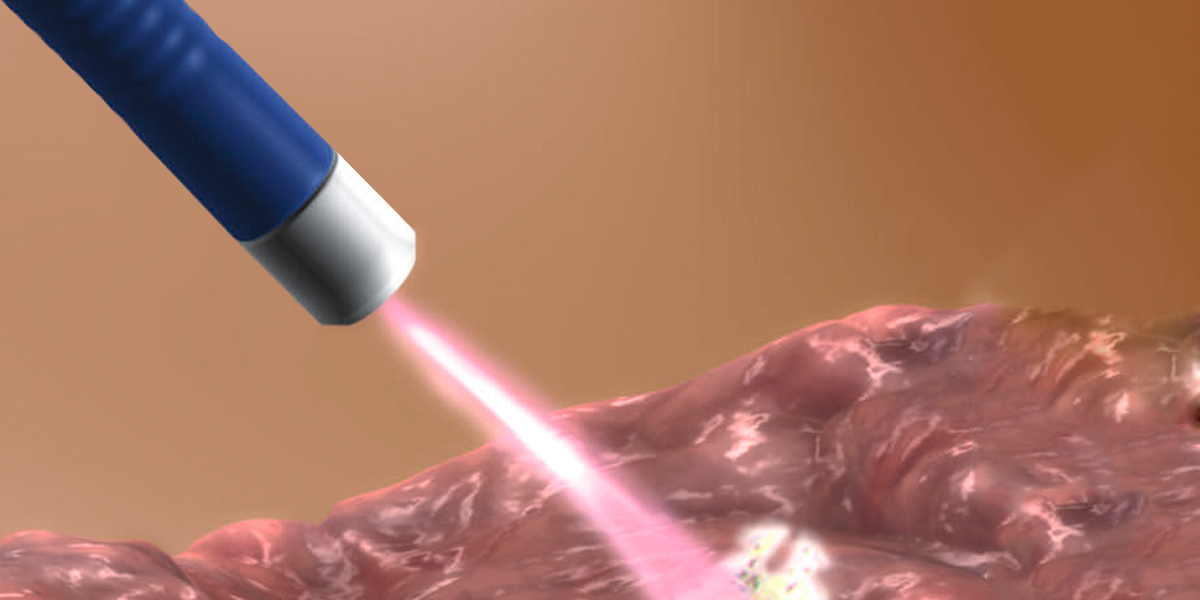
WHAT IS APC? Contrary to a common misconception, argon plasma coagulation (APC) is not a laser. This technology uses argon gas to deliver plasma of evenly distributed thermal energy to a field of tissue adjacent to the probe. A high voltage spark is delivered at the tip of the probe that ionizes the argon gas as it is sprayed from the probe tip in the direction of the target tissue. Argon gas is non-flammable and inexpensive to refill. It is easily ionized by the 6000 volt peak energy delivered by the tungsten wire( like that which was used to illuminate the olden traditional electric lamb) that terminates just proximal to the probe tip. This ionized gas or plasma then seeks a ground in the nearest tissue, delivering the thermal energy with a depth of penetration of roughly 2 to 3 mm. The plasma (ionized Argon gas) coagulates both linearly and tangentially. By delivering energy to all tissue near the probe tip, APC can be used to treat a lesion around a fold and not clearly in view or a lesion that cannot be positioned directly in front of the endoscope.
EQUIPMENT
The disposable probes are available with diameters of 1.5 mm, 2.3 mm (the most commonly used size), and 3.2 mm. The standard probes are 220 cm long, 300 cm probes can be specially ordered for use during push enteroscopy.
In addition to the probe, the equipment consists of an electrosurgical generator that comes on a cart along with the argon plasma coagulator, a foot pedal, and two tanks of argon gas. Both the argon flow rate and the wattage delivered per pulse are easily adjusted by pressing buttons on the control panel. Newer models of this cart will include a water pump.
TECHNIQUE
Argon plasma coagulation (APC) is easy to perform and can generally be learned in a few sessions. No special safety precautions are necessary for the operator.
When the endoscopist identifies
a lesion suitable for APC, a grounding pad is placed on the patient's thigh or shoulder. The argon gas from the tank is turned on and the generator and coagulator power is switched on. A flow rate (generally 0.8 to 1.0 L/min) of argon is selected.
The power setting on the APC2 current generator is adjusted based upon the location of the lesion. Lower settings, in the range of 20 to 30 watts, are used in thinner regions of the gastrointestinal tract, such as the colon and small bowel. Higher settings (30 to 40 watts) are used for the thicker-walled stomach and for tumor ablation. Depth of penetration during coagulation depends upon the wattage and the number of pulses at a particular location. Areas of previous coagulation have greater impedance, so subsequent pulses favor adjacent uncoagulated tissue. However, deeper penetration may occur from extra pulses in the same area.
Before inserting the probe into the biopsy channel of the endoscope, the operator presses the purge button on the coagulator to prime the probe with argon.
It is important not to fire too close to the target. If the probe touches the mucosa directly when firing, the coagulation is direct rather than via ionizing plasma and a deeper injury similar to monopolarelectrocautery results. Care should still be exercised to avoid coming into direct contact with the tissue.
The probe can be used to coagulate discrete lesions with isolated pulses or may be used to "paint" areas with multiple lesions (eg, in the treatment of gastric antral vascular ectasias or radiation proctopathy). Painting is performed either by dragging the endoscope with the probe in a fixed position or by swinging the probe by deflecting the endoscope tip in a pendulum fashion. Charring of the probe is generally less than is seen with contact thermal devices, but may occur when direct tissue contact is made. Occasionally, glucagon may be helpful to reduce bowel motility and facilitate treatment of multiple lesions.
Care should be taken to avoid excessive insufflation, particularly in the colon
. While there is less smoke generated than with laser therapies, it can still obscure visualization. Intermittent suction serves to prevent over-insufflation while also clearing the field of view.
Superficial ulceration occurs following APC, which typically heals within two to three weeks. Despite theoretical safety advantages due to reduced depth of penetration, all of the complications that have been reported with other thermal hemostasis techniques can occur. (See 'Complications' below.)
EFFICACY FOR GASTROINTESTINAL BLEEDING
A report from the American Society for Gastrointestinal Endoscopy concluded that APC was best suited for hemostasis of diffuse superficial vascular lesions, such as gastric antral vascular ectasia syndrome and radiation induced proctopathy.
Angiodysplasia — The anatomy and superficial location of angiodysplasia make them well suited for APC treatment. Immediate hemostasis rates range from 85 to 100 % and treatment can result in long-term control of bleeding.
Special considerations warrant comment regarding APC for angiodysplasia. Bowel motility can be problematic, particularly in the small bowel.APC does not work well if the targeted lesion is under water.
A number of recommendations can be made based upon clinical experience:
●Adjust wattage downward for targets with thinner walls.
●Calibrate the optimal distance to avoid undesired contact coagulation.
●Treat the center of the angiodysplasia.
●Frequently suction gas to avoid over-insufflation, particularly in the colon.
●Avoid getting the probe tip wet. Press the purge button when this happens.
●Wash the lesion to identify the bleeding site, but realize that APC is difficult for lesions that are under a pool of water.
Watermelon stomach/GAVE syndrome
Serial treatments with APC can reduce
transfusion requirements and raise hemoglobin in patients with gastric antral vascular ectasia (GAVE) syndrome. Most patients required three to four sessions to achieve the desired clinical benefit. However, long-term follow-up data are limited.
Radiation telangiectasias
(also referred to as radiation proctopathy or radiation proctitis.
All visible lesions were targeted at each session and follow-up procedures were scheduled in four-week intervals to allow the tissue to heal. Some patients experienced post-procedure rectal pain and cramps, but no major complications occurred.
Special care is required to avoid spraying too close to the dentate line. 5-ASA suppositories and/or Cortifoam enemas are often used to help treat procedure-induced rectal ulcers, which occur in more than one-half of patients but are usually asymptomatic. Residual ulcers seen during a follow-up examination should be avoided during subsequent treatment.
Piecemeal polypectomy
APC has been used to coagulate the base of large sessile polyps, which can eliminate residual adenomatous tissue and can assist in the control of minor bleeding.
Tumor debulking and bleeding
APC is widely used as an alternative to laser and bipolar cautery to debulk tumors, to treat tumor ingrowth in stents, and to control generalized bleeding from tumors
Care should be taken to avoid excessive insufflation, particularly in the colon
However, it has an advantage compared with other methods to temporarily control bleeding since it can rapidly coagulate a relatively wide area.
Ulcer hemostasis Endoscopic treatment of serious ulcer bleeding with active spurting or non-bleeding visible vessels is usually accomplished with mechanical or thermal tamponade techniques, with or without epinephrine injection. APC appears to be effective as part of combination therapy for visible vessels.
Esophageal varices
Limited preliminary experience suggests a possible role of APC combined with variceal band ligation in the treatment of esophageal varices. (See "Endoscopic variceal ligation".)
Dieulafoy's lesions — A case series described successful treatment of Dieulafoy's lesions (mainly in the upper gastrointestinal tract) with APC alone.
COMPLICATIONS
Argon plasma coagulation (APC) has a theoretical safety advantage over other modalities due to its decreased depth of penetration and the tendency for the ionized arc of electrical current to deflect away from coagulated tissue to surrounding areas. However, like any coagulation method, serious complications can occur, particularly in the right colon. The perforation rate was 6 out of 2193 sessions (0.2 percent). The authors reported 10 minor complications, most commonly subcutaneous emphysema. Isolated perforations have occurred in other series.
Other complications that have been observed are asymptomatic and symptomatic pneumoperitoneum and subcutaneous bubbling of gas. It is likely that these problems are caused by either over-distension within the cecum or unintentional direct contact of the probe to the thin bowel wall during pulses resulting in deeper monopolar cautery. No treatment is generally required when the subcutaneous bubbling of gas is observed.
SUMMARY AND RECOMMENDATIONS Endoscopists
Experienced with argon plasma coagulation (APC) generally find it to be helpful for targets that are difficult to reach by direct contact and for treating multiple lesions at the same session. In addition, APC tends to be quicker than contact thermal therapies. There have been few trials comparing APC to other endoscopic methods used for similar indications. The availability of the necessary equipment and familiarity with specific methods are important considerations when choosing options. We suggest APC be used in the following settings (Grade 2C):
●Targeted therapy of isolated angiodysplasia in the colon, small bowel, and stomach
●Treatment of gastric antral vascular ectasia
●Treatment of radiation telangiectasia with significant or symptomatic bleeding
● Postpolypectomy for large sessile polyps to fulgurate the base to reduce recurrent adenoma
● Ablation of flat residual duodenal or colon polyps in hard to reach locations that cannot be removed with a snare
●Treatment of tumor ingrowth into esophageal metal stents


 info@utopiapharma.com
info@utopiapharma.com
 Plot No. (2) Industrial Zone (A7) - formerly Zizinia - Cairo - Ismailia Road - 10th of Ramadan - Sharkia
Plot No. (2) Industrial Zone (A7) - formerly Zizinia - Cairo - Ismailia Road - 10th of Ramadan - Sharkia